Products You May Like
An independent advisory panel to the Centers for Disease Control and Prevention on Tuesday recommended that all Americans ages 6 months and up receive updated Covid vaccines from Pfizer and Moderna, the next step toward the shots reaching Americans in the coming days.
Thirteen advisors voted in favor of that “universal” recommendation for Americans, while one voted against it.
CDC Director Mandy Cohen still has to sign off on that recommendation before the new shots become available at pharmacies, local health departments and clinics nationwide. The CDC expects the first doses to be available within 48 hours of that recommendation, Georgina Peacock, head of the CDC’s immunization services division, said during the advisory meeting on Tuesday.
“I think that it’s clear that vaccination is going to prevent serious illness and death across all age groups. It is a vaccine-preventable disease,” said Dr. Beth Bell, clinical professor at the University of Washington and member of the panel, during the advisory meeting. “And so, for that reason, I favor the universal recommendation.”
However, some advisors argued that the CDC should only recommend the shots to people at high risk of getting severely sick with Covid, including those who are frail, older and immunocompromised.
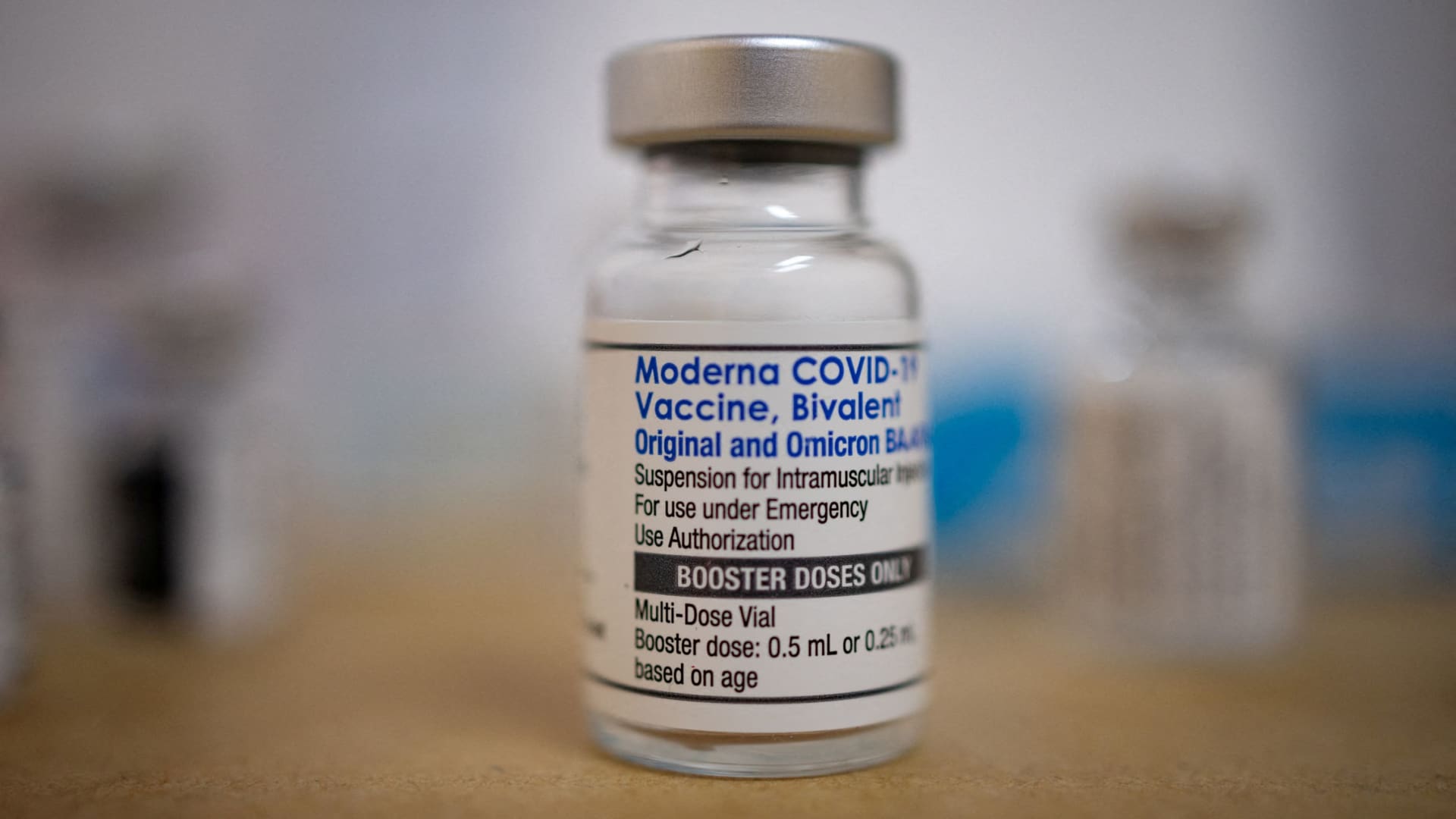
The advisory panel’s “universal” recommendation comes a day after the Food and Drug Administration approved the two mRNA jabs, which are designed to target the omicron subvariant XBB.1.5. An updated shot from Novavax, which uses protein-based technology, is still under review by the agency.
The FDA approved Pfizer and Moderna’s new vaccines for people 12 and older. The agency authorized the shots under emergency use for children 6 months through 11 years old.
The new shots are part of a push by public health officials to update Covid vaccines annually to target more recent strains of the virus – a similar approach to the yearly flu shot. The jab rollout comes as the virus starts to take a stronger hold in the U.S. again.
Hospitalizations have increased for seven straight weeks, and rose more than 15% to 17,418 for the week ending Aug. 26, according to the latest data from the CDC. That number remains below the surge the nation saw in summer 2022, when hospitalizations climbed to more than 40,000.
But the CDC “anticipates further increases” as the U.S. enters respiratory virus season this fall and winter, which is when Covid, respiratory syncytial virus and flu tend to spread at higher levels, CDC epidemiologist Dr. Megan Wallace said during the meeting on Tuesday.
The updated vaccines are expected to help prevent people from getting seriously ill and being hospitalized from Covid infections caused by newer variants.
Pfizer, Moderna and Novavax have all released initial trial data suggesting that their new shots produce robust immune responses against the now-dominant EG.5, or “Eris,” variant. That omicron strain is closely related to XBB.1.5 and accounted for 21.5% of all U.S. cases as of Sept. 2, according to the CDC.
Both Pfizer and Moderna have also released initial trial data indicating that their new shots were effective against another omicron variant called BA.2.86. Novavax on Monday said it was still testing its vaccine against BA.2.86. That strain has been detected in small numbers across the U.S., but health officials worldwide are watching it closely due to its high number of mutations.
Moderna’s XBB.1.5 vaccine “provides a substantial increase in responses to both the variant in the vaccine, and cross-neutralization of other variants – and this is regardless of prior infection status,” said Dr. Fran Priddy, executive director of clinical development for the Covid vaccine program at Moderna, during the advisory meeting Tuesday.
The upcoming arrival of the new vaccines comes months after the end of the U.S. Covid public health emergency.
The end of that declaration means manufacturers will sell their updated shots directly to health-care providers at more than $120 per dose in the private market. Previously, the government purchased vaccines directly from manufacturers at a discount to distribute to all Americans for free.
During the advisory meeting, Moderna said the list price of its vaccine is $129 per dose and Pfizer said the list price of its own shot is $120 per dose. Meanwhile, Novavax said its list price is $130 per dose.
The vast majority of Americans will be able to get the new vaccines at no cost through private insurance or government payers like Medicare.
For the uninsured or underinsured, the Biden administration plans to offer shots for free through its temporary “Bridge Access Program” at health centers, clinics and eventually pharmacies across the U.S. Free vaccines through the program will not be available after December 2024, according to the CDC’s website.
The CDC’s Vaccines For Children program will provide free Covid shots to children whose families or caretakers can’t afford them after the shots move to the commercial market.