Products You May Like
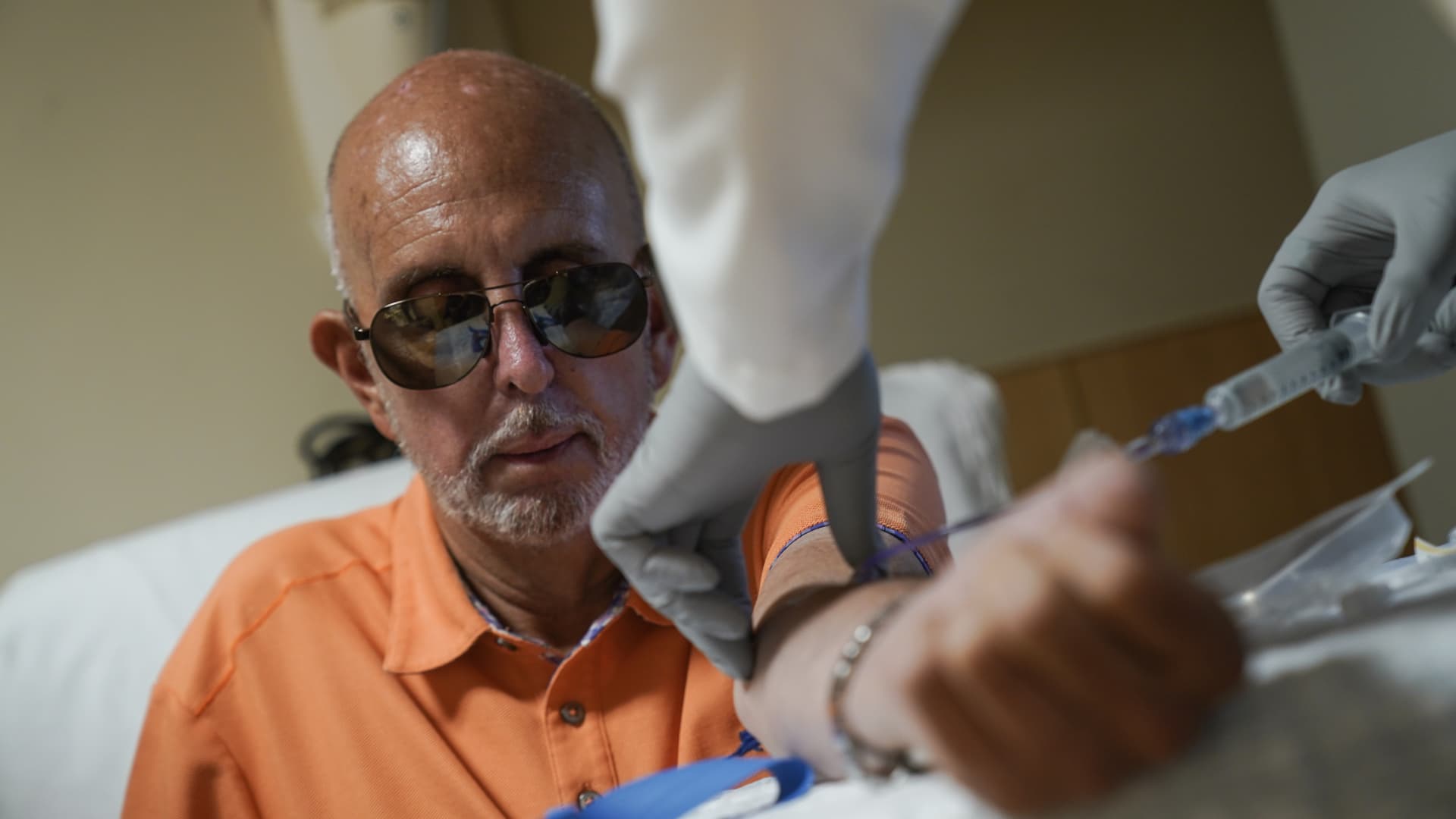
Sales of the Alzheimer’s drug Leqembi may be slow initially due to logistical requirements but could pick up in 2024, analysts said after the groundbreaking treatment won approval in the U.S.
Wall Street is chewing over the Food and Drug Administration’s Thursday approval of Leqembi – a milestone in the treatment of the disease, even though the drug isn’t a cure.
Leqembi, from drugmakers Eisai and Biogen, is the first medicine proven to slow the progression of Alzheimer’s in people at the early stages of the memory-robbing disease.
Medicare on Thursday announced it is now covering the antibody treatment for patients enrolled in the insurance program for seniors, broadening access for those who can’t afford the drug’s hefty $26,500-a-year price tag. But coverage comes with several conditions.
Analysts believe certain Medicare requirements and new guidance on Leqembi’s prescription label could potentially weigh on sales of the drug – at least in the near term.
“While logistic hurdles make accessibility to the drug challenging for the incoming 6-12 months, we do expect to start seeing sales ticking up starting in mid-2024,” Guggenheim analyst Yatin Suneja wrote in a note Thursday.
Medicare will pay for Leqembi as long as patients find health-care providers participating in a registry or a database that tracks the drug’s benefits and risks.
The initial process of building out a registry is one logistical hurdle that “will take time and could be somewhat burdensome early on,” Jefferies analyst Michael Yee said in a research note Thursday.
Yee added that the firm’s channel checks suggest doctors see the registry requirement “as a potential real-world challenge – at least in the initial phase.” But he noted that it could ease as the drug’s launch progresses.
Another hurdle could be related to a testing requirement on the drug’s prescribing label.
The FDA recommends doctors test patients for a genetic mutation known as ApoE4 before starting treatment. Those with that mutation are at greater risk of swelling and brain bleeds if they take Leqembi. About 15% of people with Alzheimer’s have ApoE4, according to the National Institute on Aging.
The testing requirement makes the drug “even more difficult to prescribe,” Stifel analyst Paul Matteis wrote Thursday.
“The strong suggestion to test, for most clinicians, is going to add another hurdle” on top of other “substantial infrastructure requirements,” he wrote.
That includes navigating Medicare’s registry requirement and coordinating PET scans and MRIs to screen for dangerous side effects of the drug.
Jefferies’ Yee also highlighted MRI monitoring – a requirement on the drug’s prescribing label – as another logistical challenge in the near term.
The label says patients should get multiple MRIs during the first year of treatment to check for signs of ARIA, a side effect that causes brain swelling or bleeding and can be fatal in rare cases.
Yee said scheduling MRI scheduling and reimbursements take time and noted that there is a fixed capacity for MRI equipment and scans.
The prescription label requirements won’t impact the uptake of Leqembi overall because “physicians were already planning to treat patients accordingly anyway,” SVB Securities analyst Marc Goodman wrote Thursday.
But Goodman, like other analysts, also noted that “we continue to expect a slow ramp in 2023 and acceleration moving into 2024.”